This is an old revision of the document!
Table of Contents
Mourad Project
BBB
Delivery of SPMNMs to CNS of rodents with artificially provoked epileptic seizures
I. Introduction
Our research group is formulating a manifold strategy, according to which, first, the SPMNMs aggregates will be given an aspect ratio (i.e. rods) to allow orientation of the sub micron rods, under the effect of a μT magnetic moment. This orienting effect requires the use of a simple homogeneous magnetic field (parallel field lines) with low intensity, the kind of magnetic fields used found in epileptic focus. Resorting to the use of magnetic moments instead of magnetic force (geometrical gradient) is more likely to match the scenario met when pyramidal cells are propagating seizure like electrical activity. Delivery of SPMNPs to CNS has been demonstrated using polymeric coating that are not reliable from a magnetic performance point of view, nor are the delivery techniques that rely on disruption of the BBB using focused ultrasound, due to the infectious risque that it entails. Part of our research hypothesis thus is the feasibility of bare SPMNMs through the BBB using static magnetic fields generated by Niodim-Yag permanent magnets attached to the head of the rodent at the time odf the intravenous delivery of the theragnostic agent.
II. Motivation • Can any shape SPM NM reach the CNS without polymeric coating and under the effect of an external magnetic field. • Answering this question is laying out the way for delivery technologies using the MRI's system strong and static magnetic field. • Could submicron rods be oriented in under the μT neuronal magnetic field • Magnetic moment driven aggregation of SPMNMs requires parallel field lines ( neither convergent nor divergent thus no specific region of the brain is concerned). • One new property is that nanorods exhibit is the fact that they are the most appropriate for use in presence of a magnetic field such as those present in an MRI system. • Experimental work will be run on rats inoculated with epileptic precursors and no symptomatic conditions like disrupted BBB or blood hypotension
III. Hypothesis
When seizures take place, μT magnetic moments are able to orient the nanorods. Whereas in the rest of the brain, the fluid shear forces dominate and electric currents due to normal brain activity produce transient magnetic field that have no noticeable effect on the aggregation coefficient of NPs nor on the orientation of the NRs. Part of this study is to quantize the aggregation coefficient at the epileptic focus using rotational (rods & moments) and translational (particles & gradients) forces.
1) A static magnetic field applied on the scalp will allow delivery of SPMNM to CNS 2) The ratio of fluids shear forces on the SPM Poly/Met nanorod's surface to the magnetic moment generated by internal currents flow in pyramidal cells will depend on the position of the nanorod relatively to the epileptic focus.
IV. Materials and methods
Given well characterized SPM nanoparticles and SPM Polmeric or Metalic nanorods suspension in water.(purchased materials). In order to mimic MRI contrast agents like Gd(III) a concentration of 5 mg/m has to be prepared by dilution. We will carry out the following experiments based on t size and dispersion.
4.1 In vivo experiments on the delivery of SPMNMs to rats CNS in submerged in a MRI static magnetic field
The following experiment is conducted first using head mounted magnets. The delivery of particles to the brain due to magnetic influence on the BBB or the complex (BBB and Nps) will be elucidated. MRI will show hypointese regions in rat brains that account for successful delivery of the NPs. In the second series of experiments, rats that were injected with the NPs in the past and that showed a history of epileptic activity following inoculation with epileptic triggering compounds, will be subjected to MRI protocol at different periods of time. Depending on the number of seizures, the aggregates will be more or less detectable. The focus will reveal its self after a right number of Nps has been deposited or trapped by the magnetic fields it harbours. Further studies are envisaged to explain the mechanism of retention of the NPs at the site of the focus. particles that will not deposit by the μT magnetic agglomeration will be cleared or biodegraded to join the naturally occurring brain iron in its fate.
4.2 Parameters • Intensity and location of static magnetic field of the permanent Niodim-Yag magnets • SPMNPs magnetic moment and size and crystal structure • Polymeric SPMNRs asperct ratio and porosity and density of magnetic cores • Metalic SPMNRs asperct ratio, criystal structure and magnetic moment • number of injection times • number of epileptic seizes
5. Magnetohydrodynamic effect
MICRO MAGNETOHYDRODYNAMICS
Key words: water molecule, cyclic water molecule, superparamagnetic nanoparticles (SPMNPs), microfluidics, SPMNPs internalization by cells.
Application: 1. Explaining why a static external magnetic field (from permanent magnet) will help SPMNs cross the Blood Brain Barrier (others use ultrasound to disrupt BBB, very dangerous) 2. No one has explained the internalization of SPMNPs by biological cells. In our case, SPMNPs should be internalized by cells because epileptic magnetic fields are produced by intracellular component of the electric field. The extra-cellular is of no use for trapping the SPMNs. Hypothesis
Water molecules bond to each other and form cyclic water under the effect of weak and time varying magnetic (or strong but not both) fields. Cyclic water exhibits super-fluidity, this is why water present in all sorts of cells (everywhere) in the body to transport nutrients and wastes in and out of every body cell. Now, when in presence of SPMNPs, water that surrounds a NP becomes ordered and confers the NP with its super fluidity properties, thus internalization of the magnetic NP by cells is facilitated. It is as if, the right magnetic excitation of the SPMNs creates a natural biocompatible coating that confers the particle with a sort of stealth behaviour, long sought by scientists, and by doing so enhances the NPs bio-distribution.
Experimental setting In microfluidic chanels where SPMNPs are positioned permannatly, depending on the presence or absence of the excitatory external magnetic field, water flux will change according to whether or not SPMNPs are magnetized or not. If theey are then microfluid (water) will spend less time between inlet and outlet of the apparatus, this will prove that water becomes superfluid around the NP (changes molecular conformation) and theus encounters lss and less resistance in the chanel.
{{:resources:p2:magnetohydro.jpg|}}
Figure 1. (a) Effect of supperfluidity of water around spherical sperparamagnetic NPs.(b) fluid properties in absence of magnetic effects
Epilepsy
MRI imaging of superparamagnetic particles and rods aggregates in the brain Application to epileptic theragnostics
I. Introduction Detection of iron oxide nanoparticles agreggates in the brain is a routine procedure achieved successfully by Finlay et al. Strangely, Finlay used T1 relaxation time of the protons, whereas T2 is the most commonly used for MRI detection of SPM Iron Oxide in the RES system (liver, spleen, lymph nodes). T1 contrast agents are different from T2 contrast agents in a sense that T1 agents have a locale effect on protons (only the protons that get into close contact with the high spin density element of the contrast agent that experience T1 shortening). In this perspective, encapsulation of T1 agents is inappropriate, because encapsulation limits proton (water) access to the high spin density element of the T1 agent. On the contrary, encapsulation (or confinement) of T2 contrast agents enhances the T2 shortening effect because T2 effect is not local. A sphere of 50 µm radius is under the effect of T2 shortening.
II. Purpose • SPM nanoparticles are proven to cross the BBB and to have high deposition coefficient in brain tumors in-vivo. No evidence in the literature shows that Nanorods can cross the BBB and make its way to the neuronal tissues. • No non functionnalization nanomaterials where used in detection of epileptic foci based on interaction of seizure's micro-Tesla fields ans nanomaterial's magnetic moment. • If we use polymeric nanorods instead of pure iron-oxide nanorods We will have a larger radius if the SPMIOs are confined to a volume of the polymer fibber. The T2 effect is remote and reaches far beyond the 50 µm boundary. And T1 sequences are aplicable due to infiltration of protons into the nao-polymeric-rod. • The larger the number of NMR signal sources is, the larger the amplitude of the signal is. • MRI sequence sensitive to water diffusion (diffusion imaging) is susceptible to create Diffusion coefficient maps of the brain. The presence of Nanorods aggregates will create anisotropic diffusion expressed in the diffusion tensor (image based). • Finally, we will investigate which of the two relaxation times is more appropriate in the brain in presence of the SPMOINPs when T2 effects are present, and to what extent is the T1 effect relevant for epileptic foci detection with nanoparticles. As for the rods (metallic or polymeric), we will optimize the diffusion gradients to become sensitive to the dynamics of diffusion of water molecules between the nanorods.
III. Hypothesis Epileptic foci Quantization of SPMIO 1) NPs, 2) Nps based fpolymeric rods and 3) SPMIO metallic rods deposits is feasible in the brain using T2 effect and diffusion maps. Inter-Ictal detection of epileptic seizure focus will be tagged by SPM nanomaterials aggregate that interacted with the μT magnetic fields generated in the Ictal phase .
IV. Materials and methods
Relaxation time (T2) will be quantified by nuclear magnetic resonance (RMN) for the proton in the presence of different concentration NPS NMs. This experiment will be ran in an identical magnetic field B0 to that developed by the MRI system at Sait-Luc Hospital (3 T). the NMR spectroscopic device is available at the Chemistry department of the University of Montreal. In order to achieve SPMs NP aggregates detection, two possibilities will be explored: 1) A classical Spin Echo sequence will be implemented due to its robustness with respect to the static field inhomogeneity caused by the SPM Nms. 2) A new sequence will be developed and that is diffusion sensitive. A new kind of surface antennas could be necessary at this point, namely a metamaterial that is supposed to double the signal to noise ratio of the imaging sequences, especially when the aggregates size expected to form, following interaction of the the SPM NMs and the seizure driven μT magnetic fields, are expected to be small. Imaging tests will first be performed on a cylindrical MRI phantom made out of bovine collagen and water. In channels of arbitrary geometries, manufactured according to our needs, SPM NMs solutions at physiologically relevant concentrations, will be deposited by their direct application on the internal surface of sad channels. Application of μT fields will follow this step. The phantom will be put in the MRI system image acquisition. The control the size (aspect ratio) of the NMs and the encapsulation process of SPMNPs in the Polymeric matrices matrices (e.g. PVA) will tremendously simplify the conditions onthe performance of the MRI system.
5.1 Preliminary experiments on the functional properties of the carriers
Proton NMR spectroscopy at 3 T • Without SPMNP (chemical shift, intensity signal ) • In presence of SPM NPs , Poly/Met nanorods( Off resonance effect, correlation sphere VS dose & size )
5.2 Spectroscopy and detection the resonance frequency of the SPM Nps, Poly/Met nanorods at 3T • Dose dependent frequency shift (effect of concentration and aggregate size) • sensitivity
5.3 MRI of a physiologically relevant dose of SPM Nps, Poly/Met nanorods in a gel phantom
• Preparation of the gel (bovine collagen or agar gel) • Use of high aspect ratio of SPM rods and Pol/Met nanorods • Adjustment sequence parameters • Perform post-acquisition image analysis of diffusion weighted imaging
5.4 Parameters SPMNPs concentration in aerosols, gel water content 5.6 Graphics expected • Spectra of protons and SPM NMs resonances at 3 T • Relaxivity of the SPM NMs on proton 1/T2 verses SPM NMs concentration • Images of SPM NMs aggregates • diffusion maps of the brain
5.7 Expected results • Quantification of aggregates by MRI using T2 images of proton • Evidence of an off-resonance effect of SPMNPs on proton resonance • translation of diffusion coefficients into surgical coordinates. • Correlation with histopathological findings and intended epilepsy triggering zones
Magnetophoresis & Simulation
Simulation of superparamagnetic nanomaterial interacting with epileptic seizure-like driven uT magnetic fields
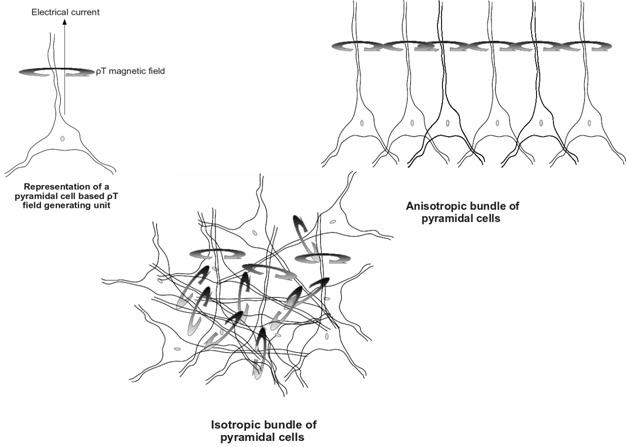
I. Introduction It is much more efficient to model the system before starting any in-vivo or in-vitro protocol. This will reduce the cost and help develop an efficient design tool, either for the imaging protols (size and morphology of the aggregates) or for the superparamagnetic materials to be used. Computer Simulation provides a means to virtually test the the interaction of the sad SPMNMs with neuronal bundles of different degrees of anisotropy (Fig. 1). It is Obvious that the isotropic configuration results in an overall magnetic field that is null, hence the impossibility of measuring it at a distance, like its the case in MEG. As a mater of fact, MEG detects magnetic fiels developed by highly anisotropic configurations inherent to temporal and hypocampal regions of the brain. The aggregation of superparamagnetic nanomateriarls will thus, address the tow configurations identically, since the SPMNMs are assumed to be omnipresent in the brain at the ictal and interictal periods of time. So close they will be from the sources of the electrical currents propagating in the pyramidal cells, that they will be under the influence of 1) pico-Tesla fields in the isotropic case and 2) micro-Tesla fields in the anisotropic one.
Figure 1. Tow different magnetic fields configurations II. Time independent analysis we will start with a modeling approach based on finite element modeling FEM to model SPMNMs absence of motion in presence of hydrodynamic forces and naturally occurring magnetic fields like the ones found in an epileptic seizure focus. An epileptic focus can be likened to a trap for the nanoparticles that eventually one has to asses its trapping efficiency. To do so, the neuronal network configurations will be considered independently from the particles and their motion. Using a static simulation that takes into account, magnetophoretic and hydrodinamic forces in their analytical forms, one can easily identify the spacial coordinates of regions where the forces add up to zero [1]. this simulaion work could be carried out on simulation platforms combining Matlab or ANSYS.
III. Time dependent analysis
Conventional simulation tools like ANSYS and COMSOL are not adapted to our application since they excel in modeling cases where applied fields are strong and thus, they consider their propagation unaffected by particles shapes and varying magnetic moments. In case of weak magnetic applied fields, the propagation of the magnetic field will be sparse and transient non uniform field are created within the particle itself, let alone at inter-sources distances. Consequently, particles shapes and magnetic moments are critical to this effect, and redefining the propagation of the magnetic fields is mandatory, using a whole new technique that combines MATLAB to calculate and define the time varying proprieties of nanoparticles and insert them in the ANSYS geometric model, to iterate on the magnetic and hydrodynamic constraints. The derived transient forces from iteration step J, will be used to calculate the spacial position/and/or angular rotation for iteration step J+1. The magnetophoretic and hydrodynamic loads for the next iterations are recalculated and forces are derived for the new simulation step. IV. Theoretical considerations The paramagnetic particle (aspect ratio L/D) subjected to a magnetic field has a magnetic moment m proportional to its magnetic susceptibility tensor where is the magnetic volume of the particle. Projecting along its main axes and with , it writes :
If we replace the anisotropy of susceptibility of the rod by and if the magnetic field rotates (abruptly or at a pulsation ) in the plane , the rod experiences a magnetic torque:
and a viscous torque [1]:
Where is the coefficient of hydrodynamic drag experienced by the rod and the instantaneous angular velocity of the rod, n, the viscosity of the fluid carrier, L the length and D the diameter of the nanoparticle respectively. References
[2] A. Anguelouch, R. L. Leheny, and D. H. Reich, “Application of ferromagnetic nanowires to interfacial microrheology,” Appl. Phys. Lett. 89, 111914 (2006).
Interferometry
Optical Characterization Of A Cantilever Array : Toward Integrated Wave Front Correctors And Analyzers For Miniaturized Adaptive Optics Systems
This paper presents the design, optimization and optical characterization of an array electrostatic actuators based MEMS to be used as an adaptive optics component of a portable retinal imaging device. The proposed wave-front corrector is implemented in (CMC) polymump technology and features an array of cantilevers on which planar reflective gold thin films were deposited for characterization purposes. The cantilevers relative deflections will be set based on the output of a wave-front analyzer to correct higher order optical aberrations. For the sake of achieving precise wave front correction, three cantilever sizes were implemented on the chip for a total of 64 cantilevers that will serve as actuators of a deformable mirror in future work. Array of actuators are hindered by the off-plane initial misalignment phenomenon caused by thin films deposition process and mismatches between the cantilevers displacement due to size nonuniformities introduced by the fabrication process. The device total surface is XXX × XXX mm2. The COMSOL simulation of the modeled Large, medium and small size cantilevers predicted maximum deflections of 6, 4 and 2 um respectively at maximum DC voltages of XXX, XXX and XXX respectively. These values are verified by laser interferometry and shown that they can be considerably augmented by omitting the gold deposited layer in future versions of the device.
Brain surgery
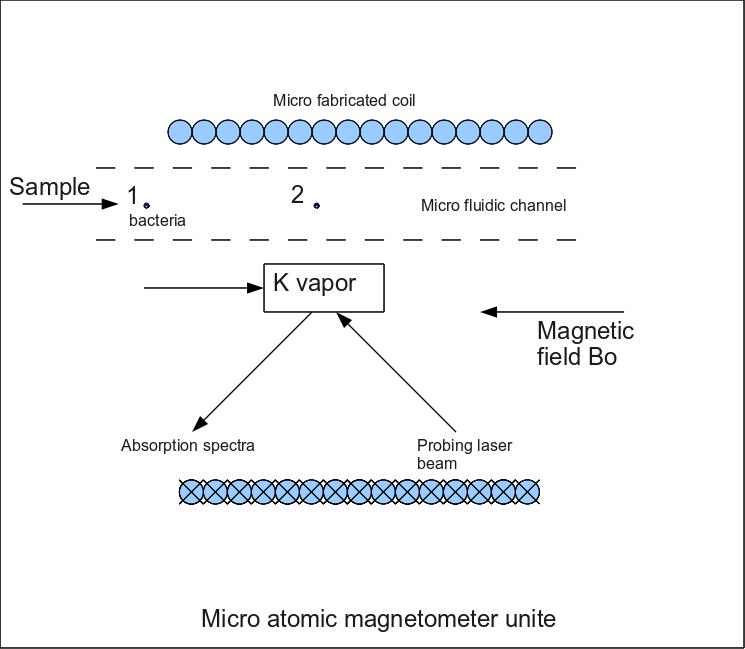
I. Brain surgery project The scale of miniaturization achieved in microfabrication and the paralleled and unprecedented accomplishment in low cost and low energy CMOS IC fabrication technology, are not exploited to the fullest extent, and remain good candidates for the ultimate solution of online detection of cancer cells, with no need for cumbersome and inaccurate MRI systems. The ultimate goal of this research activity is to develop a miniaturized (Micro-device), magneto-optical sensor, accurate enough to detect the lowest density of cancer (glioblastoma) cells, in the brain. The idea real time detection of cancer cells has been approached in different ways. Recently, photo-fluorescent-guided surgical sampling of gliobalastoma, was used to phenotypically distinguish tumor-initiating cells in the tumor mass and margin, but in vain [1]. Literature pertaining to the field of detection of cancer cells, gushes with articles emphasizing the need for early detection of cancer cells colonies at early stages of development, in general and their real time detection during ablations or surgical resection,in particular. The morbidity that glioblastoma entail emphasizes the need for ultra sensitive means of differentiating healthy from diseased cells, so that the resection margins are left with a safe number of malignant cells, that could be eventually dressed with chemotherapeutic agents. It has been evidenced that a reliable detection method should use high field MRI systems (say; 8T) using folic acid functionalized iron oxide superparamagnetic SPMNPs. More sophisticated methods relying on SQUIDs (superconducting quantum interference devices) devices have been proven more efficient when trying to detect cell colonies of a few hundreds of carcinoma cells, while affordable 1.5 T MRI systems are only capable of detecting huge colonies of carcinoma cells, tagged with SPMNPs (say; few thousands of cells). But the real-time aspect can only be implemented in open MRI system, to allow the surgeon to enter the MRI system along with patient and the surgical staff. The later aspect defeats the purpose of sensitive detection, since open magnets develop weak magnetic fields their images exhibit low signal to noise ratios. On the other hand, the cost and availability of open MRI systems makes them inadequate for development of point of care therapies. This is where atomic magnetometers come into play. It is hypothesized by our research group that a micro system, that harbors an microfabricated atomic magnetometer, is capable of Zero-field (earth magnetic field) detection of glioblastoma cells, during excision of the tumor, provided the cells are previously tagged with functionalized SPMNPs. The microfabricated magnetometer is to be mounted aboard the surgical tool used for excision.
1.2 Atomic magnetometer Atomic magnetometry is more sensitive than SQUIDs (Superconducting Quantum Interference Devices made nowadays in micrometer size by micro fabrication of Josephson Junction). Based on electromagnetically induced transparency of alkali metal vapors, for example, potassium vapor (electron resonance EPR) placed in a magnetic field Bo (be it earth's magnetic field), becomes opaque to a probing laser beam when it couples to NMR (nuclear magnetic resonance) signals of protons (brain water) or EPR (electron magnetic resonance) signals of tumor bounded, SPMNPs, provided the frequency of the probing beam is set to the potassium electron transition frequency that corresponds to the NMR or EPR frequencies of protons and SPMNPs respectively. The quantification of the number of cells is performed by integrating the curves from laser absorption spectra. 1.3 functionalization of nanoparticles Many polymeric compounds have been proven to cross the blood brain barrier (BBB) like PVA (polyvinyl Alcohol ) when synthesized at special molecular weights and Oilicon Oxide SiO2 to name a few. Except that when PVA or SiO2 are functionalized with Anti-bodies for tumor detection, the complex (SPMNP/coating/Anti-body) looses its stealthiness and becomes detectable by the BBB. 1.4 Injection of SPMNPs Instead of using a topical application during surgery in order to tag cancerous cells and washing them out after photo-luminescent sensing, our research group suggests the investigation of the (BBB) preparation (heightened permeability). In the literature, BBB sonication has been applied on mice was proven efficient in allowing functionalized polymeric nanoparticles to reach the brain. Never has the technique been studied on human subjects. The other option is to inject the markers beyond the BBB.
Figure 1. Schematic of tumor detection unite