Table of Contents
1. Lipid Bilayer Sensor Array
Priority: April 20, 2009
Abstract
• You have an array of sensor elements.
• You have a bunch of detection channels capable of amplifying electrical signals from sensor elements.
• More sensor elements elements are provided than detection channels.
• Detection channels selectively connected to sensor elements.
• Connect only to sensor elements with acceptable performance quality.
• This improves efficiency of utilization of the detection channels.
• Results in reduction of cost and ability to perform sensing using relatively small samples.
1.1 Outline
• [0002] To perform sensing of molecular entities, it has been disclosed to use membrane proteins inserted in a lipid bilayer.
• [0005] The weak nature of the signals will require a separate detection channel for each sensor element which impacts cost.
• [0019] By selectively connecting the detection channels 30 to respective sensor elements it is possible to increase the efficiency with which the channels are used.
• [0020] The increase in efficiency arises due to the ability to select sensor elements that have acceptable quality of performance, for connection to a detection channel.
• [0025] This invention differs from time-division multiplexing (TDM) since its intend involves the redundant provision of sensor elements whose quality of performance varies. Thus events can happen faster than switching (unlike TDM) because the intention is to continuously attach to well performing sensors (once they are identified). The trouble must stem from the fact that the quality of sensor performance may be quite variable due to the need to form bilayers and insert member proteins which can vary every time such sensors are constructed (and construction might be done every few hours or even minutes I am guessing).
1.2 Sensor
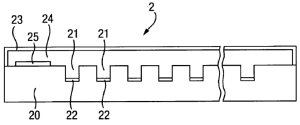 • [0044] Imagine the sensor device 2 consists of a large number (256 to 1024 typically) wells 21 with electrodes 22 arranged therein.
• [0044] Imagine the sensor device 2 consists of a large number (256 to 1024 typically) wells 21 with electrodes 22 arranged therein.
• [0046] Bilayers are formed across the wells and membrane proteins (i.e. nanopores) randomly inserted into each well's bilayer. Such spontaneous insertion is a dynamic process and so there is a statistical variation in the number of membrane proteins inserted into individual lipid bilayers, typically having a Poisson distribution.
• [0048] In the example below, acceptable quality of performance is the insertion of a single effective membrane protein, With plural membrane proteins being unacceptable. In other situations, insertion of plural effective membrane proteins may be acceptable.
1.3 Apparatus
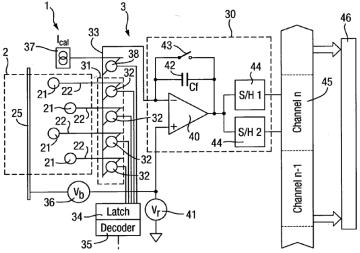
• [0049] The wells are divided into groups. The picture uses 4 wells per group as an example. One detection channel per group is used. For some applications the sensor device might comprise a total of 4096 wells 21 and 1024 detection channels 30.
• [0050] The apparatus includes a switch arrangement 31 which is capable of selectively connecting the detection channel to any one of the wells in the group. In particular the switch arrangement is a 1-to-4 multiplexor comprising 4 switches each connected to one well electrode 22 and a common contact 33 which is itself connected to the input of the detection channel 30.
• [0051] The switches 32 are preferably semiconductor switches, preferably field effect transistors. The switches are selected to provide minimal leakage to the detection channel either from the wells that are not connected through the switches that are open or from the latch 34 through the switches. Dynamic charge injection effects are avoided by running the apparatus 1 with the switches in a static configuration for most of the time.
• [0052] Any one single switch 32 is closed at a time.
• [0053] There is no requirement to be able to change the configuration of the switch arrangement 31 rapidly. Typically, changes may be required on a time-scale of minutes and a complete update should be achievable on a timescale of up to 0.1 s to l s.
• [0059] The detection channel 30 includes a charge amplifier 40 that is a differential amplifier.
• [0061] The output of the charge amplifier 40 is connected to two sample-hold amplifiers 44 arranged in parallel and optionally provided with voltage gain. The S/H amps are used to provide correlated double sampling. Each S/H is switched synchronously with the control switch 43. The S/H output goes to a multiplexor 45 which supplies the date to a processing unit (assuming A/D conversion is done somewhere in between).
• [0062] Alternatively can use two charge amplifiers 40 in parallel.
1.4 Sensor Selection Process [0068] - [0072]
• Measure signals to choose some well in each group. Facility to try again if no sensor found or if a once good sensor loses quality.
• [0072] However, it is noted that the quality of performance of wells 21 becoming unacceptable is relatively rare.
1.5 Redundancy and Efficiency [0073] - [0090]
• [0074] The insertion of membrane proteins into a lipid bilayer 26 is a random process that follows Poisson statistics. This means that even when the average number of membrane proteins per well 21 is one, a significant number of wells 21 may have none, two or more membrane proteins inserted, and these wells 21 are then not useful. For example, it is found that in a particular embodiment the maximum probability for finding just one membrane protein in a well 21 is about 36%, and this is only achieved if conditions are optimal. A greater or lesser membrane protein concentration quickly results in a reduction of useable wells 21 (especially a lesser exposure). Current estimates for efficiency which is likely to be achieved in practical embodiments are about 20%.
• [0076] Lipid bilayers 26 are formed with an efficiency which may be assumed to approach 100% for current purposes. Membrane proteins are then inserted using a solution whose concentration and exposure time is adjusted to give a mean number of membrane proteins per well 21 near to one. Because the wells 21 might not in practice have a lipid bilayer 26 of the same size, their capture efficiencies will vary. This combines with Poisson statistics to give a spread in the number of membrane proteins per well 21. The apparatus 1 characterises the sensor device 2 to detect which wells 21 have active, useful membrane proteins.
• [0078] The mean of the pore Poisson distribution depends on
- area of the lipid bilayer
- concentration of membrane proteins in the solution
- time for which lipid bilayer is exposed to the membrane protein solution
• [0087] Cross-bar switch is possible, but much more complex.
1.6 Modified Detection Circuit [0091] - [0098]
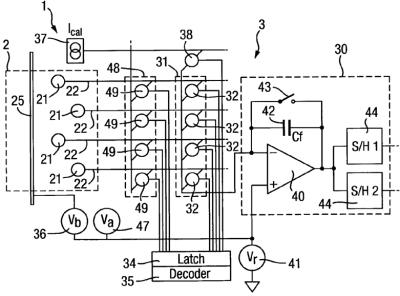 • [0092] In this one you can unblock the pores (supply an inverted potential) without affecting other wells 21.
• [0092] In this one you can unblock the pores (supply an inverted potential) without affecting other wells 21.
• [0095] Now provide a second switch arrangement 48 that can connect up the unblocking bias source 47 to the pore in question.
• These switches are referenced in Dec. 01, 2009 patent.